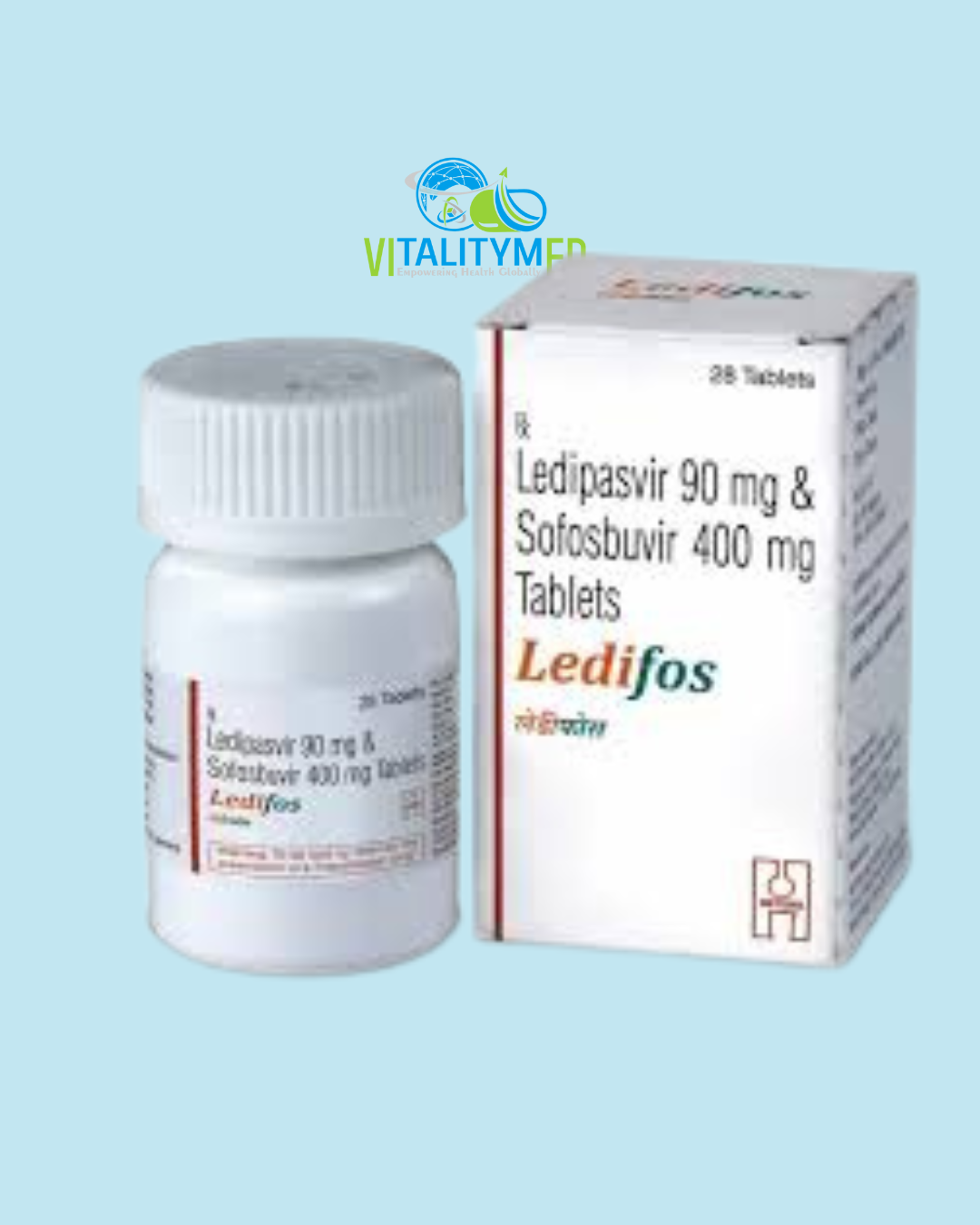
Ledifos combines Ledipasvir and Sofosbuvir, two direct‑acting antiviral drugs formulated in a single oral tablet. It is FDA‑approved (under the brand name Harvoni) for treating chronic hepatitis C virus (HCV) infection, particularly genotypes 1, 4, 5, and 6, in adults and children aged 3 and above—with or without compensated cirrhosis. It’s also used in certain cases with decompensated liver disease, often combined with ribavirin.
Mechanism of Action
-
Ledipasvir inhibits the HCV NS5A protein, disrupting essential processes involved in viral replication, assembly, and secretion.
-
Sofosbuvir is a nucleotide analog that targets NS5B RNA polymerase, acting as an RNA chain terminator once incorporated into viral RNA. Together, they produce a synergistic antiviral effect.
Uses
Ledifos is prescribed for:-
Treating adults and children (≥ 3 years) with chronic HCV genotype 1, 4, 5, or 6 infection, regardless of cirrhosis status.
-
Treating genotype 1 infection in patients with liver transplant or decompensated cirrhosis, often combined with ribavirin.
-
An effective option for HCV/HIV co-infected patients under compatible antiretroviral regimens, showing cure (SVR12) rates around 96–98 %.
Adverse Effects
Common side effects (in > 10 % of patients):-
Fatigue, headache, weakness; also insomnia.
-
Nausea, diarrhea, rash, and cough occur in 1–10 % of people.
Serious warnings:
-
Risk of reactivation of hepatitis B virus (HBV) in co-infected patients, which can cause liver failure or death. Consultants should be screened before starting therapy.
-
Risk of bradycardia or slow heart rate when used with amiodarone, sometimes with fatal outcomes. Requires monitoring.
-
-