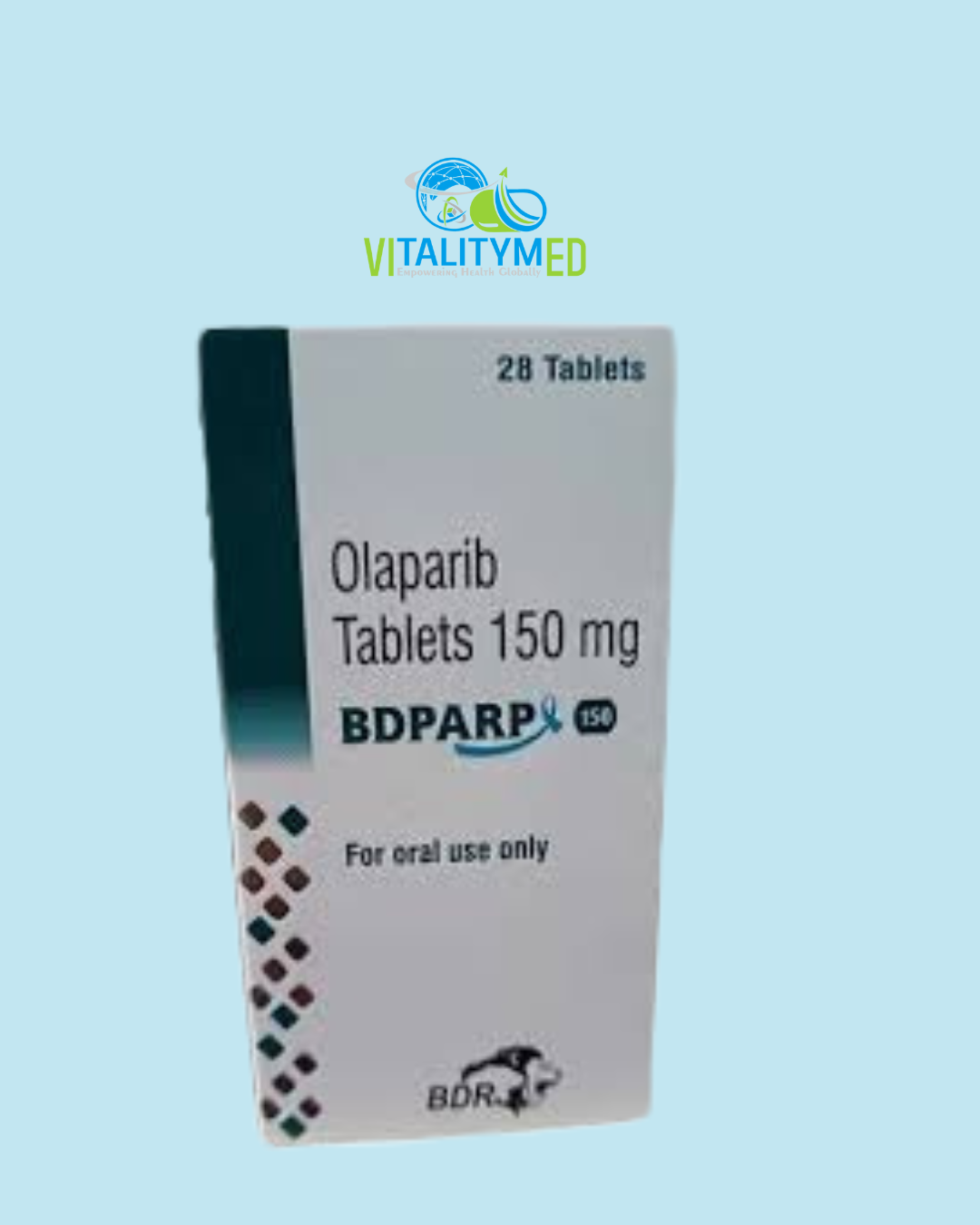
BDPARP contains olaparib, an oral anticancer medication belonging to the class of PARP inhibitors. It is used mainly for the treatment of certain cancers with genetic mutations such as BRCA1 or BRCA2, particularly in the ovaries, breast, pancreas, and prostate. By targeting a specific weakness in cancer cells, olaparib helps slow or stop the progression of tumors while aiming to minimise damage to healthy tissues.
Mechanism of Action
Olaparib works by inhibiting an enzyme called poly ADP ribose polymerase or PARP, which plays a key role in repairing damaged DNA. Cancer cells with BRCA mutations already have impaired DNA repair mechanisms. When PARP is blocked by olaparib, these cells are unable to repair their DNA effectively, leading to cell death. This process is known as synthetic lethality, where the inhibition of multiple repair pathways selectively kills tumor cells while sparing normal ones.
Uses
BDPARP is approved for use in various cancers, often in patients whose tumors have specific genetic mutations. These include advanced ovarian cancer, especially after response to platinum-based chemotherapy, metastatic breast cancer with BRCA mutations, pancreatic cancer, and prostate cancer with homologous recombination repair deficiency. It is typically used in maintenance settings or when standard treatments are no longer effective.
Adverse Effects
Common side effects of olaparib include fatigue, nausea, vomiting, decreased appetite, and abdominal pain. Some patients may experience anemia or other blood-related issues such as low white blood cell or platelet counts, which require monitoring. Less frequently, patients may develop shortness of breath or signs of lung inflammation. Rare but serious risks include the development of secondary blood cancers such as leukemia or myelodysplastic syndrome. Regular blood tests and clinical reviews are necessary to ensure safe and effective treatment.