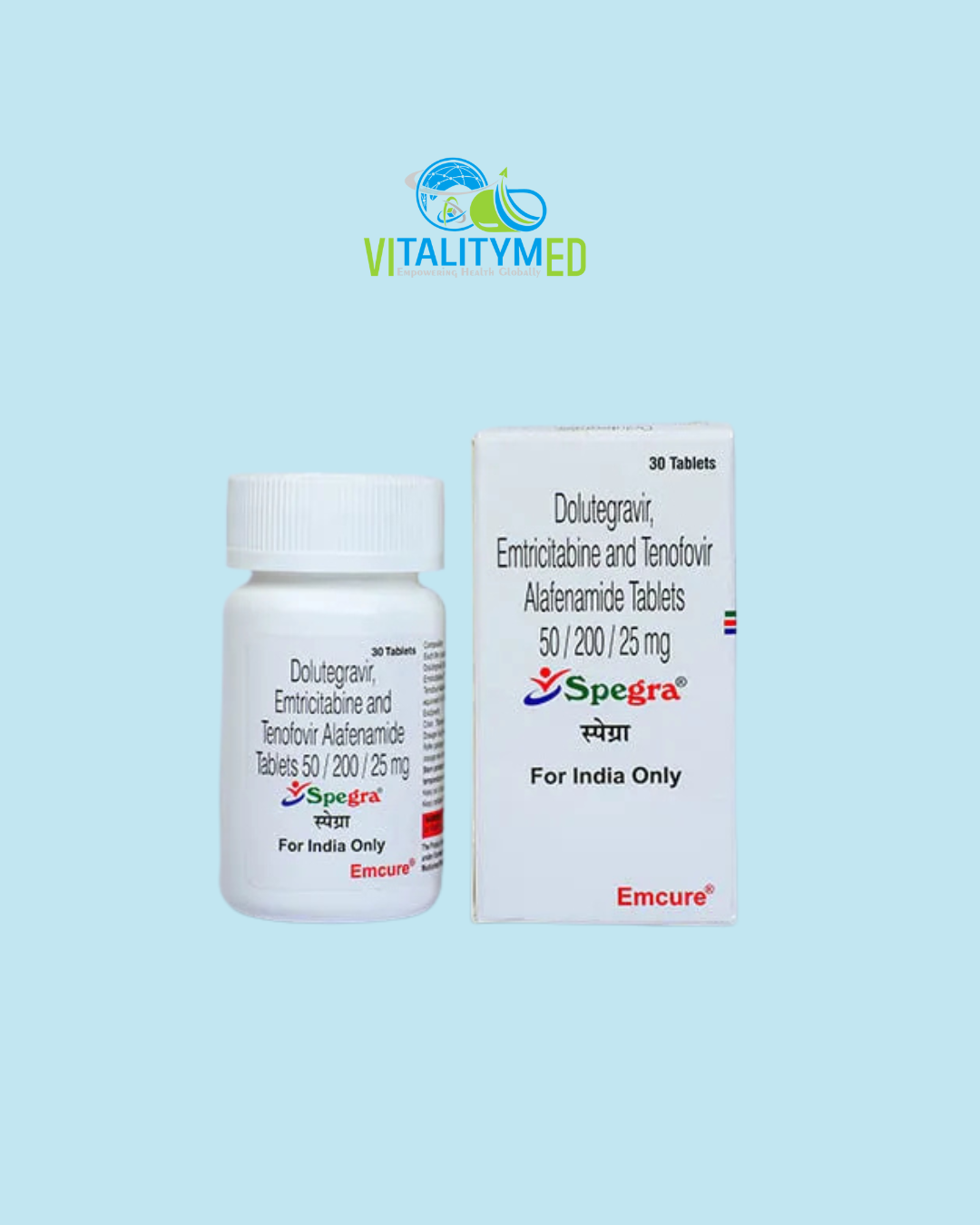
Spegra is a once-daily fixed-dose combination tablet that contains three antiretroviral agents: tenofovir disoproxil fumarate, emtricitabine, and dolutegravir. It is used in the treatment of HIV-1 infection and offers a complete regimen in a single pill, improving convenience, adherence, and long-term viral control. Spegra combines two nucleoside reverse transcriptase inhibitors (NRTIs) with an integrase strand transfer inhibitor (INSTI), making it a highly effective and widely recommended option for both newly diagnosed and treatment-experienced patients.
Mechanism of Action
Each component in Spegra works in a distinct way to stop the virus from multiplying:
-
Tenofovir and emtricitabine are NRTIs. They mimic the natural building blocks of DNA and get incorporated into the viral DNA during replication. Once inserted, they block further chain growth, preventing the virus from copying its genetic material.
-
Dolutegravir is an INSTI. It inhibits the enzyme integrase, which the virus uses to insert its DNA into the human cell’s genome. Without integration, the virus cannot reproduce.
Uses
Spegra is indicated for the treatment of HIV-1 infection in adults and adolescents weighing at least 40 kilograms.
It is commonly used for:-
First-line therapy in individuals who are newly diagnosed with HIV
-
Switching from other regimens in stable patients for better adherence or tolerability
-
Ongoing lifelong therapy to keep the virus suppressed and protect immune health
Adverse Effects
Spegra is generally well tolerated, but as with any medication, side effects can occur.
Common side effects include nausea, headache, tiredness, diarrhea, and sleep disturbances. Some people may also experience dizziness or mood changes such as anxiety or depression.Dolutegravir can cause a mild increase in creatinine levels, which may affect kidney function tests without causing real kidney damage. Rarely, serious allergic reactions or liver toxicity may occur, especially in individuals co-infected with hepatitis B or C.
Tenofovir, especially in long-term use, may rarely affect kidney function or bone mineral density. Regular monitoring is recommended to catch these issues early.
-