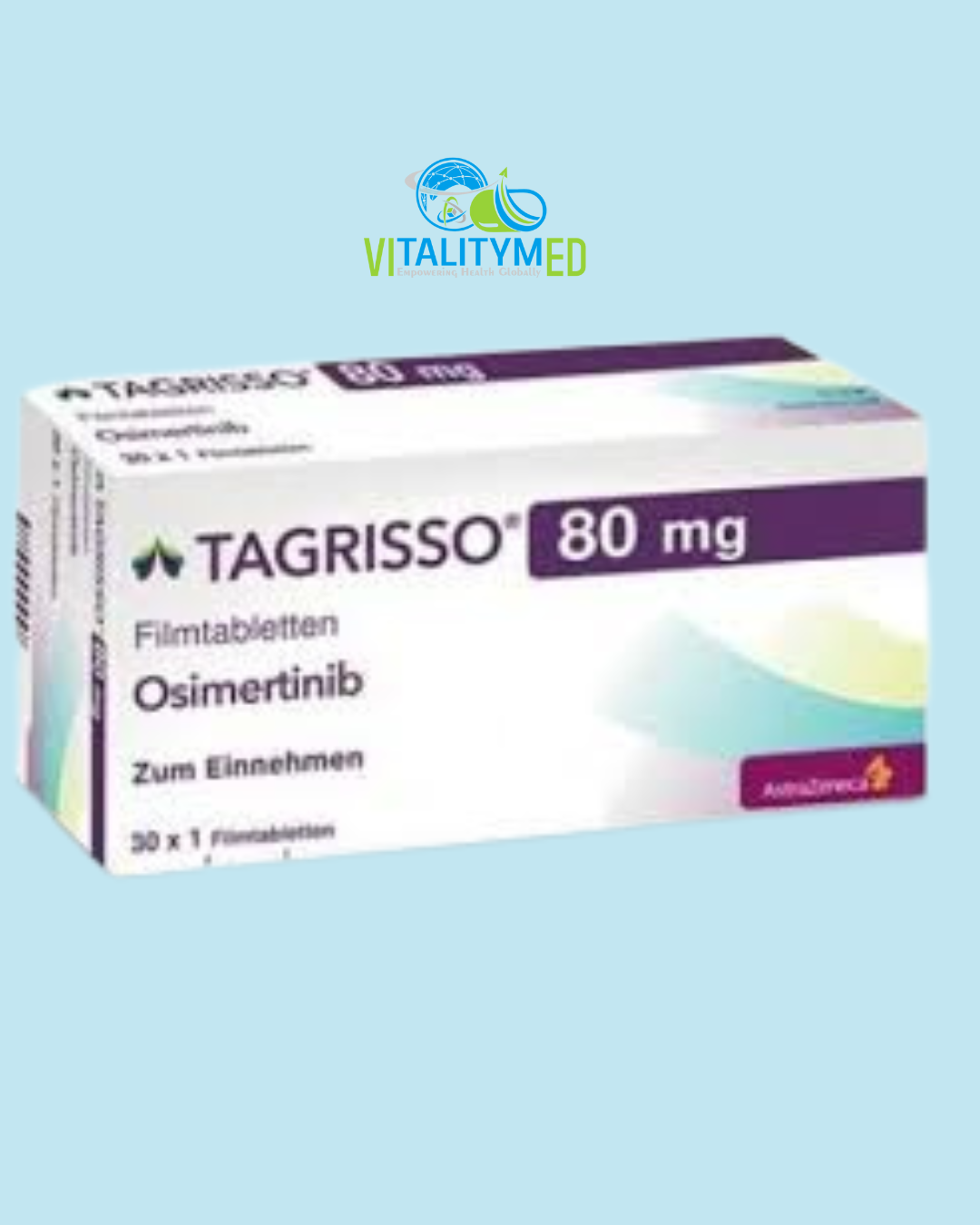
Tagrisso contains osimertinib, a targeted therapy used in the treatment of certain types of non-small cell lung cancer. It belongs to a class of drugs known as tyrosine kinase inhibitors and is specifically designed to act on cancer cells with particular mutations in the epidermal growth factor receptor. Osimertinib has become a key option for patients with advanced lung cancer who have tested positive for these mutations, offering a more precise and often better tolerated treatment approach.
Mechanism of Action
Osimertinib works by selectively inhibiting mutant forms of the epidermal growth factor receptor that drive cancer cell growth and survival. These mutations cause the receptor to stay permanently activated, sending continuous signals that promote uncontrolled cell division. Osimertinib binds to and blocks this abnormal signalling, leading to reduced tumour growth and, in many cases, tumour shrinkage. Importantly, it is also effective against the T790M mutation, a common cause of resistance to earlier generation EGFR inhibitors.
Uses
Tagrisso is used for the treatment of adults with non-small cell lung cancer that tests positive for EGFR mutations. It is approved both for initial treatment of advanced EGFR mutation-positive lung cancer and for use in patients whose cancer has progressed after treatment with other EGFR-targeted therapies and has developed the T790M resistance mutation. It is also used as adjuvant therapy in early stage lung cancer to help reduce the risk of recurrence after surgery.
Adverse Effects
While generally well tolerated compared to traditional chemotherapy, osimertinib may cause side effects. Commonly reported issues include diarrhoea, rash, dry skin, and nail changes. Some patients may experience tiredness or a decrease in appetite. Less commonly, serious lung problems such as pneumonitis, heart rhythm abnormalities, or eye disorders can occur. Liver function changes and low blood cell counts may also be observed and require regular monitoring during treatment. Prompt reporting of unusual symptoms to the healthcare team is essential for safe use.